Late-stage functionalisation – defined as “a desired chemoselective transformation on a complex molecule to provide at least one analog in sufficient quantity and purity for a given purpose without the necessity for installation of a functional group that exclusively serves the purpose to enable said transformation” [1] – has emerged as a valuable enabling tool within the arsenal of a medicinal chemist. As newer synthetic methods become developed for the generation of small molecules, so too has the chemical space that medicinal chemists will have access to.
Of course, these methods are valuable to the R&D team – but did you know that late-stage functionalisation will also likely have an impact on the IP profession? This perspective provides some insight into how late-stage functionalisation not only helps the chemists at the bench, but also the patent attorneys working in-house and within outside counsel who are very much off the bench.
The conventional approach to chemical synthesis – the “building block” route
Traditionally, the procedure for installing the required functionality in a small molecule typically involves utilising starting materials that already include such groups, or conducting one or more reactions that generate such groups – this stitching together of starting materials to generate the final product can be termed a “building block” approach.
Schematically, one such approach could be illustrated as follows: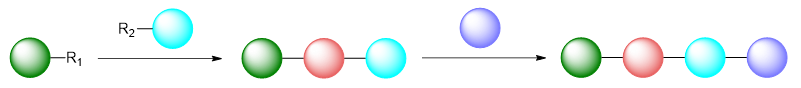
For example, a synthesis of the anticancer drug, imatinib (GLEEVEC®) might utilise the following route:
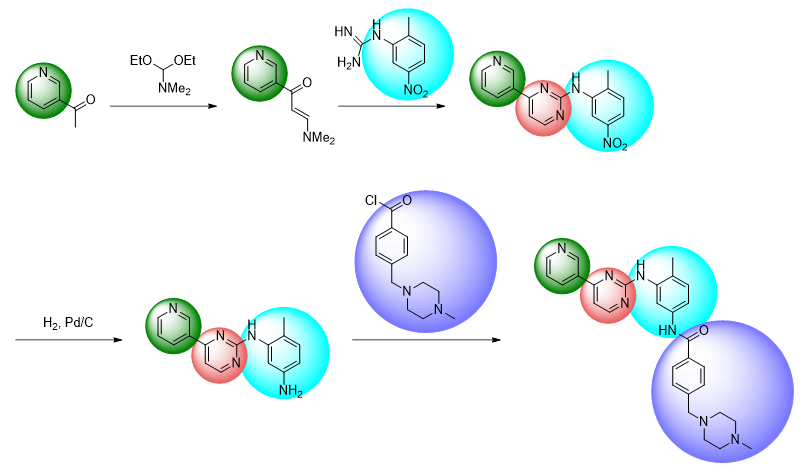
The advent of late-stage functionalisation
The discovery and development of mild, chemoselective, site-selective and stereoselective reactions has fundamentally changed the way in which chemists think about synthetic chemistry. Reactions that were once considered fanciful decades ago are now enabled by recent advancements in transition metal catalysis, photoredox catalysis and the like.
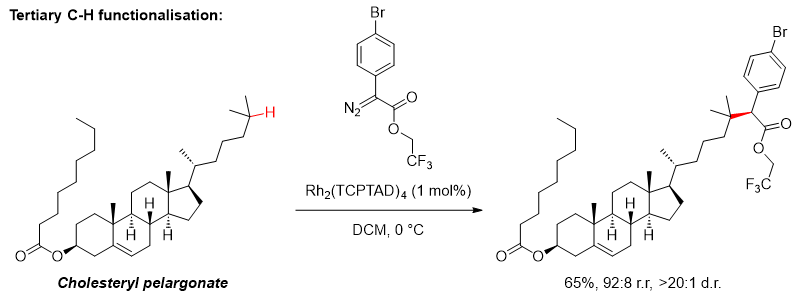
For instance, it is now perfectly reasonable to think of various C-H groups as reactive handles at which functionality can be installed by C-H functionalisation [2].
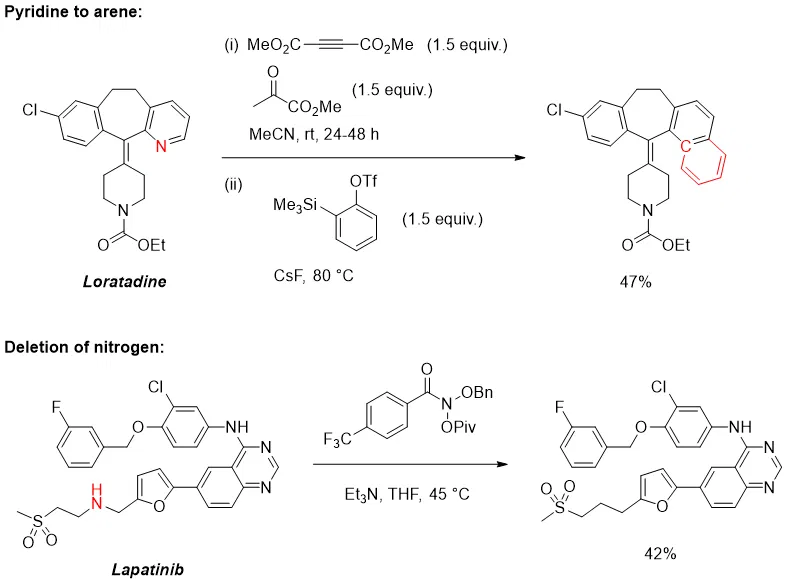
It is also now possible to simply swap out or even delete certain functional groups by so-called “skeletal editing” [3,4].
The medicinal chemist’s perspective
During the course of R&D, it is necessary to investigate structure-activity relationships (SAR). By investigating different types of substituents at various positions of a small molecule, the biological activity of the compound can be optimised.
The traditional “building block” approach can often conflict with the types of modifications desired when investigating SAR. Of course, it can be easier to investigate the effect of swapping out certain substituents if they were already introduced at a late stage of the synthetic route, illustrated schematically and with the example of imatinib below:
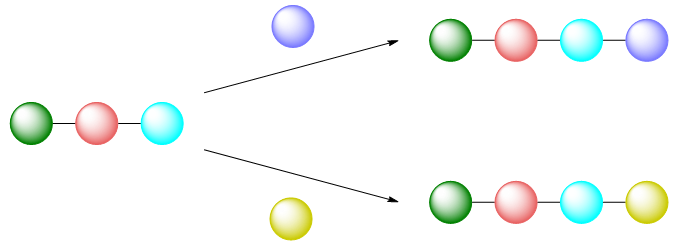
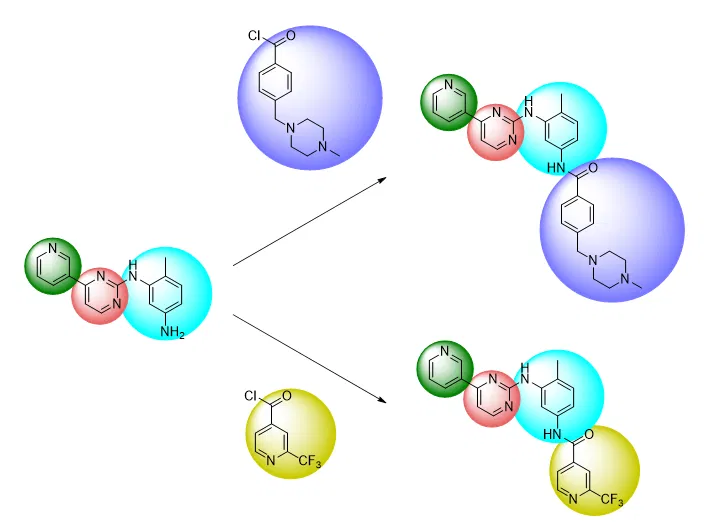
Conversely, however, it is more difficult to investigate the effect of making structural changes if the groups to be replaced were already introduced at an early stage of the synthetic route. In this case, it is often necessary to redo the synthesis with a different starting material, or in extreme cases, redesign the entire synthetic route:
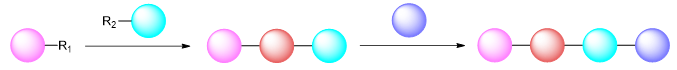
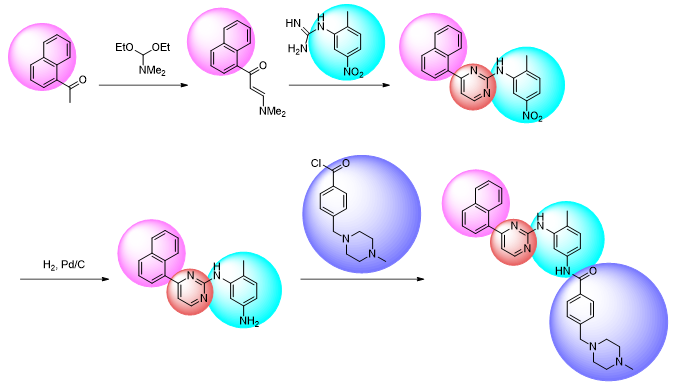
The need to perform substantial changes to the synthetic route can therefore represent a significant barrier to performing SAR studies, meaning that investigating variation at some positions of the small molecule may not be worthwhile and/or too laborious to conduct.
Late-stage functionalisation changes the playing field. Rather than starting from scratch, one can apply such techniques to modify specific groups at specific positions, in compounds that have already been synthesised before:

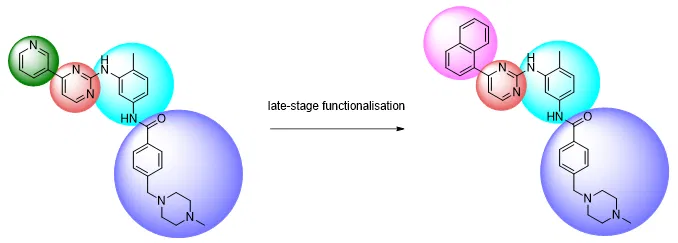
Thus, late-stage functionalisation has opened up new avenues for the medicinal chemist to explore in terms of structural variation – opportunities that may have been difficult to access or even impossible in the past.
But what about patents?
A patent application relating to small molecules will typically contain claims that refer to a general chemical formula with variables at different positions of the chemical structure – this is known as a “Markush” structure, such as the example below:
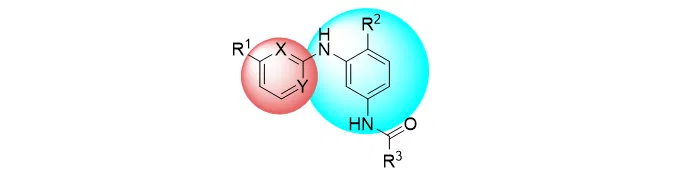
In the structure above, the substituent R1 might, for example, be defined as follows:
“wherein R1 is selected from hydrogen, halogen, OH, a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, a substituted or unsubstituted alkynyl group, a substituted or unsubstituted amino group, a substituted or unsubstituted aryl group, and a substituted or unsubstituted heteroaryl group”.
We have discussed in previous articles of our Data driven IP insights series that experimental evidence will be required showing how the invention can be reproduced by others (or, in legalese, by the notional “person having ordinary skill in the art” or “skilled person”). For a patent application relating to small molecules, this is typically achieved by providing synthetic procedures and characterisation data in the patent specification for a number of chemical compounds falling within the scope of such a Markush structure, much like the supplementary information section in a journal article.
The amount of experimental data required to “support” a claim to such a general chemical structure will very much depend on where you are pursuing patent protection.
Some jurisdictions may be relatively more lenient and will allow such claims to grant, even though not all substituent types have been exemplified by the experimental data of the patent specification (e.g. if an alkyl group has been exemplified in the examples for R1 in the Markush structure above, but not alkenyl or alkynyl groups).
However, other countries can be stricter, requiring that each substituent type be exemplified by at least one compound in the patent specification (e.g. if only an alkyl group has been exemplified in the examples for R1 in the Markush structure above, then the remaining substituents listed for R1 that are not exemplified may need to be deleted, before the patent office will allow the claim to grant). In such jurisdictions, the need to limit the scope of protection of the claims may be undesirable and can make it easier for third parties to work around the protection afforded by a granted patent.
There are distinct parallels between the situation in the medicinal chemistry space and the situation in the patent space. Much like how the traditional “building block” approach has hindered scientists from exploring various structural variations, there has also been a similar barrier to obtaining experimental data that supports broader claim scopes (e.g. trying to obtain evidence supporting other substituents for R1 in the Markush structure above) – whether this obtaining experimental data for drafting a first patent application, or obtaining such data for submission as post-filed experimental evidence in support of attorney arguments.
Advancements in late-stage functionalisation could play a key role in allowing innovators to obtain the protection they rightly deserve, after embarking on long and arduous R&D programmes for developing new clinical candidates.
Taking the Markush structure and imatinib examples above, imagine if, for the sake of argument, imatinib was the only compound in the (draft) patent specification where R1 was different from hydrogen (i.e. supporting a heteroaryl group), and all other compounds in the patent specification were cases where R1 is hydrogen. As discussed above, some jurisdictions will allow the original definition to be retained, despite the lack of experimental data to support all substituent types. Stricter jurisdictions are likely to raise objections on grounds of lack of “support”, and ask that any substituent types that are not directly exemplified be deleted, or that supplementary experimental evidence be provided showing that compounds with other types of substituents can be synthesised and retain efficacy.
This is where late-stage functionalisation might come into play. It can be difficult to obtain supplementary experimental data, especially if the compounds and/or intermediates were synthesised a long time ago and have been disposed of or misplaced, or perhaps are slowly decomposing in storage. Instead of starting from the beginning to obtain the necessary experimental data, it may be possible to start from the clinical candidate itself, and conduct late-stage functionalisation at selected positions of the chemical structure. In the Markush structure and imatinib examples above, an objection of lack of support on R1 could potentially be addressed by conducting late-stage functionalisation on imatinib (e.g. by transforming the pyridine ring to an arene ring, i.e. to support an aryl group), conducting the relevant biological assay, and then submitting such experimental data in response.
Late-stage functionalisation is therefore likely to open up new opportunities for innovator companies – we anticipate that late-stage functionalisation will not only have an impact in academia and industry, but also in commercial and IP aspects too.
- Börgel and Ritter, “Late-Stage Functionalization”, Chem, 2020, 6, p. 1877-1887
- Liao et al., “Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds”, Nature, 2017, 551, p. 609-613.
- Cheng et al., “Skeletal editing of pyridines through atom-pair swap from CN to CC”, Nat. Chem., 2024, 16, p. 741-748.
- Kennedy et al., “Skeletal editing through direct nitrogen deletion of secondary amines”, Nature, 2021, 593, p. 223-227.



